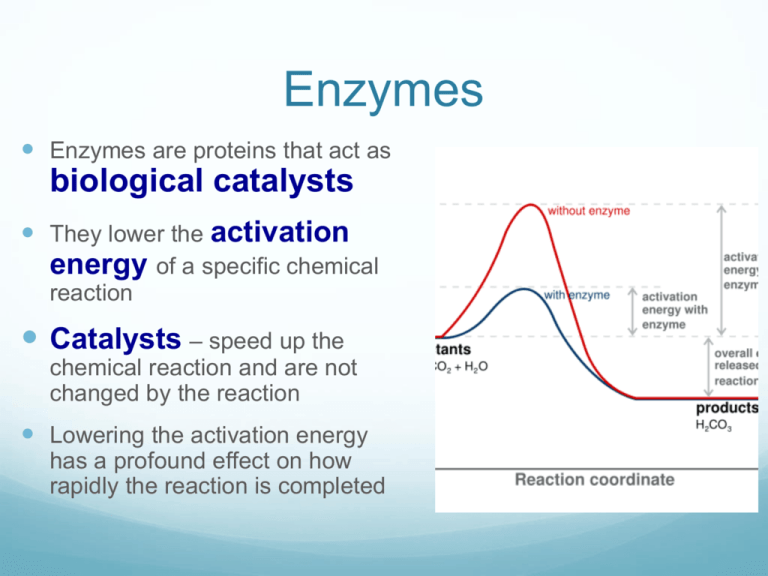
Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed . On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation. enzymes work by lowering the activation energy needed to start biochemical reactions. The activities of enzymes depend on the temperature, ionic conditions, and the ph. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction.
from cetylqdp.blob.core.windows.net
One of the ways the activation. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. The activities of enzymes depend on the temperature, ionic conditions, and the ph. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. enzymes work by lowering the activation energy needed to start biochemical reactions.
Describe How Enzymes Lower Activation Energy at Patricia Chambers blog
Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. enzymes work by lowering the activation energy needed to start biochemical reactions. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. One of the ways the activation. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. The activities of enzymes depend on the temperature, ionic conditions, and the ph.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed The activities of enzymes depend on the temperature, ionic conditions, and the ph. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT Lecture 8 Enzyme PowerPoint Presentation, free download ID6356615 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes lower the activation energy necessary to transform a reactant into a product. enzymes work by lowering the activation energy needed to start biochemical reactions. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. One of the ways the activation. enzymes can lower the. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From slideplayer.com
Enzymes. ppt download Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes can lower the activation energy of a chemical reaction in three ways. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. One of the ways the activation. enzymes work by lowering the activation energy needed to start biochemical reactions.. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Enzymes and Enzyme Activity Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. The activities of enzymes depend on the temperature, ionic conditions, and the ph. enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed One of the ways the activation. enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT 6.2 Enzymes and Chemical Reactions pages 156160 PowerPoint Presentation ID2433764 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed One of the ways the activation. enzymes work by lowering the activation energy needed to start biochemical reactions. enzymes can lower the activation energy of a chemical reaction in three ways. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction.. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From cetylqdp.blob.core.windows.net
Describe How Enzymes Lower Activation Energy at Patricia Chambers blog Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed One of the ways the activation. enzymes can lower the activation energy of a chemical reaction in three ways. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. The activities of enzymes depend on the temperature, ionic conditions, and the ph. enzymes lower the activation. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From cetylqdp.blob.core.windows.net
Describe How Enzymes Lower Activation Energy at Patricia Chambers blog Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. enzymes lower the activation energy necessary to transform a reactant into a product. the energy required. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.expii.com
What Are Enzymes? — Definition & Overview Expii Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed The activities of enzymes depend on the temperature, ionic conditions, and the ph. enzymes lower the activation energy necessary to transform a reactant into a product. enzymes work by lowering the activation energy needed to start biochemical reactions. enzymes can lower the activation energy of a chemical reaction in three ways. the energy required to reach. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From study.com
Function of Enzymes Substrate, Active Site & Activation Energy Video & Lesson Transcript Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. One of the ways the activation. The activities of enzymes depend on the. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.youtube.com
Enzymes and Activation Energy YouTube Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes can lower the activation energy of a chemical reaction in three ways. enzymes lower the activation energy necessary to transform a reactant into a product. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. On the left is a. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT BIOCHEMISTRY UNIT 1 PART 4 Enzymes PowerPoint Presentation, free download ID9135206 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would without the. enzymes work by lowering the activation energy needed to start biochemical reactions. enzymes lower the activation energy necessary to transform a reactant into a product. The activities of enzymes depend on the temperature, ionic conditions, and. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From present5.com
Enzyme Structure classification and mechanism of action Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes can lower the activation energy of a chemical reaction in three ways. The activities of enzymes depend on the temperature, ionic conditions, and the ph. enzymes work by lowering the activation energy needed to start biochemical reactions. by lowering this energy barrier, enzymes allow reactions to proceed thousands to millions of times faster than they would. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT Chapter 6 Basic Concepts of Enzyme Action PowerPoint Presentation ID2739632 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation. enzymes work by lowering the. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed One of the ways the activation. enzymes can lower the activation energy of a chemical reaction in three ways. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. On the left is a reaction that is not catalyzed by an enzyme. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID1361511 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed enzymes work by lowering the activation energy needed to start biochemical reactions. enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation. the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction.. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation. On the left is a reaction that is not catalyzed by an enzyme. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.
From www.slideserve.com
PPT Energy, Enzymes, and Biological Reactions PowerPoint Presentation ID669492 Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed the energy required to reach the transition state (the activation energy) constitutes a barrier to the progress of the reaction, limiting the rate of the reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one. by lowering this energy barrier, enzymes allow reactions to proceed thousands to. Explain How Enzymes Lower The Activation Energy Needed To Allow Reactions To Proceed.